Preparation of Oxygen
Oxygen is prepared in lab generally in two ways either by the application of heat or no application of heat.
Using heat:
Oxygen in lab is prepared by heating the mixture of powdered potassium chlorate and manganese dioxide in the ratio 4:1 in a hard glass test tube. The oxygen gas is observed in a gas jar through the downward displacement of water. The reaction involved is given below: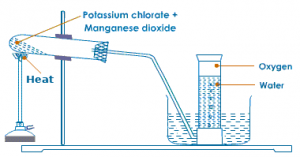
2KClO3−→−−−−−MnO2200−300∘C2KCl+3O2↑
Without heat:
The dry sodium peroxide is taken in a conical flask and the apparatus is fitted as shown in figure. The two necks of woulfe’s bottle is tighten up with cork so that no air enters from outside. It is connected with the delivery tube to a water trough containing water. Water is poured slowly from the thistle funnel. Here sodium peroxide reacts with water at ordinary temperature to give hydrogen gas. The hydrogen gas thus formed is collected in a gas jar through delivery tube by the downward displacement of water. The reaction takes place as below:
2Na2O2+2H2O⟶4NaOH+O2↑
Physical properties of oxygen:
– Oxygen is colorless, odourless and tasteless gas.
– It is pale blue color in liquid and solid state.
– It is slightly soluble in water.
– It is heavier than air.
Chemical properties of oxygen:
Combustibility: It does not burn itself but it supports for combustion. Oxygen requires high initial heating due to its high bond dissociation energy of 493.4KJmol– between the O=O atoms.
Action with hydrogen: Oxygen when heated with hydrogen forms water.
2H2+O2−→Δ2H2O2H2+O2→Δ2H2O
Action with Nitrogen: Oxygen reacts with nitrogen at high temperature to give nitrogen dioxide.
N2+O2−→−−−3000∘C2NON2+O2→3000∘C2NO
NO+O2⟶2NO2NO+O2⟶2NO2
Action with carbon: Carbon when reacted with limited oxygen gives carbon monoxide and with excess oxygen, carbon gives carbon dioxide.
2C+O2limited⟶COCarbon Monoxide2C+O2limited⟶COCarbon Monoxide
2C+O2Excess⟶CO2Carbon Monoxide2C+O2Excess⟶CO2Carbon Monoxide
Action with ammonia: Oxygen reacts with ammonia to give nitric oxide and water. It is a reaction involved in manufacture of nitric acid in Ostwald’s process.
4NH3+5O2−→−−−Pt/MO800∘C4NO+6H2O↑4NH3+5O2→Pt/MO800∘C4NO+6H2O↑
Action with glucose: Glucose reacts with oxygen to give carbon dioxide, water and energy. This reaction takes place inside the human body.
Glucose + Oxygen→Carbon Dioxide + Water + EnergyGlucose + Oxygen→Carbon Dioxide + Water + Energy
i.e., C6H12O6+6O2⟶6CO2+6H2O+EnergyC6H12O6+6O2⟶6CO2+6H2O+Energy
Action with metals:
Oxygen combines with many metals to form their respective oxides.
4Na+O2−→−−−−−−−−−Room temperature2NaO24Na+O2→Room temperature2NaO2
2Na+O2−→−−300∘CNa2O22Na+O2→300∘CNa2O2
4K+O2⟶2K2O4K+O2⟶2K2O
2Mg+O2−→Δ2MgO2Mg+O2→Δ2MgO
2Zn+O2−→Δ2ZnO2Zn+O2→Δ2ZnO
4Al+3O2−→ΔAl2O34Al+3O2→ΔAl2O3
Action with iron: Oxygen when reacted with iron gives ferrous oxide. When excess of oxygen is passed, it gives ferrosoferic oxide and further addition of oxygen gives ferric oxide. To be discused
Fe+O2−→ΔFeOFerrousoxideFe+O2→ΔFeOFerrousoxide
6FeO+O2−→Δ2Fe3O4Ferrosofericoxide6FeO+O2→Δ2Fe3O4Ferrosofericoxide
4Fe+3O2−→Δ2Fe3O3Ferricoxide4Fe+3O2→Δ2Fe3O3Ferricoxide
When oxygen is reacted with oxygen in presence of water, rust is formed.
4Fe+3O2+2H2O⟶2Fe2O3⋅XH2ORust4Fe+3O2+2H2O⟶2Fe2O3⋅XH2ORust
Uses of oxygen:
– It is used for artificial respiration in hospitals, mountaineers in high altitude, miners and sea divers in the form of oxygen mask.
– It is used as aero fuel in rocket engines and planes.
– It is used for the generation of energy inside our body.
– It is used as strong oxidizing agent in laboratory.
– It is used by the plants for the process of photosynthesis.
– It is used in preparing different explosives.
– It is used as a germicides and insecticides.
– It is the main element for the formation of ozone.